产品描述
*The optimal dilutions should be determined by the end user. For optimal experimental results, antibody reuse is not recommended.
*Tips:
WB: 适用于变性蛋白样本的免疫印迹检测. IHC: 适用于组织样本的石蜡(IHC-p)或冰冻(IHC-f)切片样本的免疫组化/荧光检测. IF/ICC: 适用于细胞样本的荧光检测. ELISA(peptide): 适用于抗原肽的ELISA检测.
引用格式: Affinity Biosciences Cat# BF0189, RRID:AB_2833834.
展开/折叠
75SirT1; hSIR2; hSIRT1; HST2, S. cerevisiae, homolog of; NAD dependent deacetylase sirtuin 1; NAD dependent protein deacetylase sirtuin 1; OTTHUMP00000198111; OTTHUMP00000198112; Regulatory protein SIR2 homolog 1; SIR1_HUMAN; SIR2; SIR2 like 1; SIR2 like protein 1; SIR2, S.cerevisiae, homolog-like 1; SIR2-like protein 1; SIR2ALPHA; SIR2L1; Sirt1; SirtT1 75 kDa fragment; Sirtuin (silent mating type information regulation 2 homolog) 1 (S. cerevisiae); Sirtuin 1; Sirtuin type 1;
抗原和靶标
Purified recombinant fragment of human SIRT1 expressed in E. Coli.
研究领域
· Cellular Processes > Cell growth and death > Cellular senescence. (View pathway)
· Environmental Information Processing > Signal transduction > FoxO signaling pathway. (View pathway)
· Environmental Information Processing > Signal transduction > AMPK signaling pathway. (View pathway)
· Human Diseases > Substance dependence > Amphetamine addiction.
· Human Diseases > Cancers: Overview > MicroRNAs in cancer.
· Organismal Systems > Aging > Longevity regulating pathway. (View pathway)
· Organismal Systems > Aging > Longevity regulating pathway - multiple species. (View pathway)
· Organismal Systems > Endocrine system > Glucagon signaling pathway.
文献引用
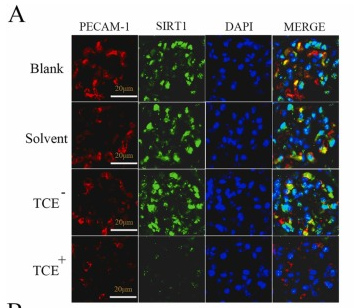
Application: IF/ICC Species: Mouse Sample: endothelial cells
Application: WB Species: Rat Sample:
Application: WB Species: Mouse Sample: liver tissues
Application: IF/ICC Species: Mouse Sample: liver tissues
Application: WB Species: Human Sample: HepG2 cells
Application: IF/ICC Species: Human Sample: HepG2 cells
限制条款
产品的规格、报价、验证数据请以官网为准,官网链接:www.affbiotech.com | www.affbiotech.cn(简体中文)| www.affbiotech.jp(日本語)产品的数据信息为Affinity所有,未经授权不得收集Affinity官网数据或资料用于商业用途,对抄袭产品数据的行为我们将保留诉诸法律的权利。
产品相关数据会因产品批次、产品检测情况随时调整,如您已订购该产品,请以订购时随货说明书为准,否则请以官网内容为准,官网内容有改动时恕不另行通知。
Affinity保证所销售产品均经过严格质量检测。如您购买的商品在规定时间内出现问题需要售后时,请您在Affinity官方渠道提交售后申请。产品仅供科学研究使用。不用于诊断和治疗。
产品未经授权不得转售。
Affinity Biosciences将不会对在使用我们的产品时可能发生的专利侵权或其他侵权行为负责。Affinity Biosciences, Affinity Biosciences标志和所有其他商标所有权归Affinity Biosciences LTD.